Introduction
Age-related Macular degeneration (AMD) is a leading cause of severe central vision loss in older adults. While treatments for the less common wet form of AMD have revolutionized care, there were no treatment options for the more common dry form for decades. Photobiomodulation is an exciting new, non-invasive treatment option for dry AMD.
How AMD Affects the Retina and Macula
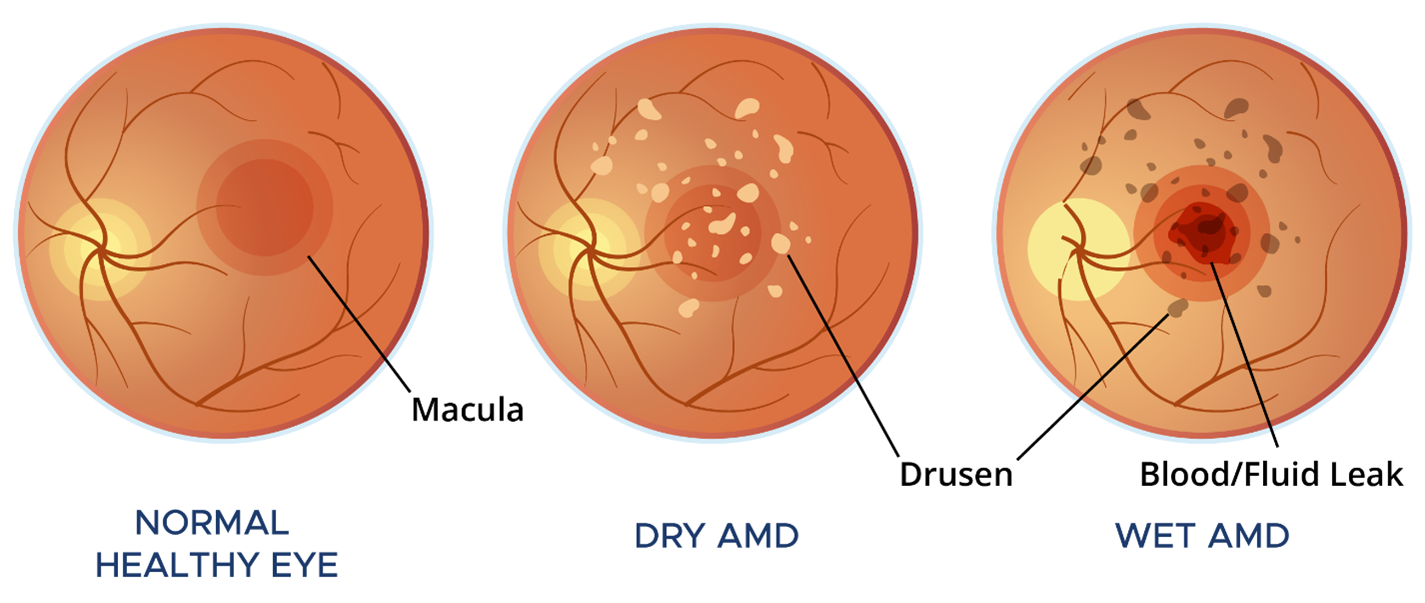
AMD damages the retina. If you think of your eye as a camera, the retina lines the back of the eye and functions like the film, sensing light and sending images to the brain. AMD specifically affects the macula—the central, most critical part of the retina responsible for sharpness and detail as well as color vision. When the macula is damaged, everyday activities such as reading, driving, and recognizing faces become increasingly difficult and, in many cases, impossible.
Dry AMD is more common and typically progresses slower than wet AMD. It affects roughly 80% of all AMD patients and can lead to central vision loss over many months or years. In contrast, wet AMD causes faster and often more severe central vision loss, sometimes developing over just days or weeks.
Dry AMD and Current Treatments
Nearly all patients with AMD initially develop the “dry” form of the disease. In dry AMD, the macula develops accumulations of debris called drusen that form in the foundational layer. Over time, patients with dry AMD may develop more drusen and lose retinal tissue in the macula. This loss of macular tissue is called geographic atrophy (GA). When GA spreads in the central macula, central vision is lost as the macula gradually “wastes away” due to dry atrophy.
Doctors monitor patients with dry AMD for drusen formation and geographic atrophy (GA), as well as for signs of wet AMD, such as blood or fluid in the retina. When patients have early dry AMD in one or both eyes, they may take a twice-daily vitamin supplement formulated by the National Eye Institute. These supplements, studied in the Age-Related Eye Disease Studies (AREDS 1 and 2), have been shown to reduce the risk of progression of dry AMD by about 25%. However, AREDS supplements do not reduce the number or size of drusen, slow the growth of GA, or provide benefit in the treatment of wet AMD.
Understanding and Treating Wet AMD
In wet AMD, abnormal blood vessels grow and leak within and beneath the foundation of the macula. This leakage of blood and fluid is what makes the condition “wet.” The leakage damages the macula and can ultimately lead to permanent scarring. Medications for wet AMD work by stopping abnormal blood vessel growth and reducing leakage to help limit further damage. Wet AMD is treated with drugs that are injected into the eye. These treatments can halt vision loss and, in many cases, even restore vision. Treatment plans are tailored to each patient with the goal of preventing leakage and further damage.
A New Treatment Option for Dry AMD
Until last year, there was no other treatment available for dry AMD beyond monitoring and AREDS supplements. After decades of retinal research, scientists discovered that light therapy, known as photobiomodulation (PBM), can stimulate retinal cells to enhance beneficial cellular activity, promote healing, and reduce inflammation. The FDA approved the Valeda® Light Delivery System (Valeda) PBM system in November 2024.

Valeda PBM uses specific light wavelengths to improve retinal cell function in the macula. The Valeda® Light Delivery System delivers three types of multiwavelength PBM treatments that act on retinal targets to improve overall retinal cell function and counter AMD-related disease processes.
What to Expect During Treatment
Valeda PBM is an in-office procedure that requires no preparation or anesthesia. Patients do not need eye drops or pupil dilation beforehand. Each treatment lasts 4 minutes and 10 seconds per eye, with the full session taking less than 10 minutes. The procedure is painless, and each eye is treated individually during the same visit. Patients receive nine treatments over 3–5 weeks, with this cycle typically repeated three times per year.
Clinical Evidence and Ongoing Research
Multiple clinical trials have evaluated the safety and benefits of Valeda PBM. After two years of treatment, most patients had stable or improved vision. Treated patients experienced a reduced number and area of drusen, slower progression of geographic atrophy, and a lower risk of vision loss. Importantly, Valeda PBM did not increase the likelihood of developing wet AMD. The system has been available in Europe for several years, and a larger study, EUROLIGHT, is currently underway to track outcomes in 500–1,000 patients across all stages of AMD. Valeda PBM provides patients with a new, painless option to help combat this vision-threatening disease.
We are excited to now offer this groundbreaking treatment for Dry AMD patients at Chester County Eye Care. To find out if it’s right for you, give us a call at 610-696-1230 or request an appointment by clicking here.
.avif)


.avif)
.avif)
.avif)
.avif)
.jpg)


%20(1080%20x%201080%20px)%20(3).png)